Clenil® Modulite® pMDI
Your doctor, nurse or pharmacist has prescribed a Clenil pMDI to help you to manage your asthma.
‘pMDI’ stands for ‘pressurised metered dose inhaler’, and your prescribed inhaler should look like one of the inhalers in the image here.
Taking your inhaler as prescribed can help to manage your symptoms, and this page contains information to help you with this.
Remember, should you need additional advice regarding your asthma, please speak to your doctor, nurse or pharmacist.

Your Clenil® Modulite® (beclometasone) inhaler may look a little different from now:
Due to a shortage in supply of dose indicators, you may have been, or may be in the future, prescribed a Clenil inhaler without a dose indicator.
What does this mean?
If you receive a Clenil inhaler without a dose indicator:
- your inhaler contains the same medication as before – there’s no change to how you manage your asthma
- your inhaler remains in the same type of device (pressurised metered dose inhaler; pMDI) – there’s no change to the way you take your inhaler.
The only difference is that, unlike Clenil inhalers that you may have received previously, there is no dose indicator to confirm how many doses remain.
What do you need to do?
- check the package label and canister of your Clenil inhaler before you use it to ensure that you have received the expected inhaler strength
- as usual, be sure to keep track of when you start to use your inhaler – each Clenil inhaler contains 200 doses of medication, so if you are taking two puffs of your inhaler twice a day, this means the inhaler will last for over a month
- if you use a VolumaticTM Spacer Device, you should continue to use this as usual
- remember to clean your inhaler once a week. You will find instructions on how to do this on the package leaflet.
Reporting of side effects: If you get any side effects, talk to your Doctor, Pharmacist or Nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the Yellow Card Scheme at: https://yellowcard.mhra.gov.uk. By reporting side effects you can help provide more information on the safety of this medicine.
Your inhaler contains a type of medicine called a corticosteroid (beclometasone) that helps to reduce swelling and inflammation within the lungs. These are also known as brown inhalers or ‘preventers’ as they help ‘prevent’ the symptoms of mild, moderate or severe asthma.
How to use Clenil
How to use ClenilClenil User Guide
Clenil User GuideClenil Patient Information Leaflet
Clenil Patient Information LeafletUnderstanding your inhaler
Your doctor, nurse or pharmacist will have one of the following Clenil inhalers. Each has a different dose and you should take your medicine as prescribed.
First use
Firstly, it’s important to read all of the Patient Information Leaflet provided with your inhaler carefully before you start using this medicine because it contains important information for you. You can download the Patient Information Leaflet using this link.
Before using your inhaler for the first time or if you have not used the inhaler for 3 days or more, 1 puff should be released into the air to make sure it works.
- Remove the protective cap from the mouthpiece
- Hold your inhaler upright with the mouthpiece at the bottom
- Direct the mouthpiece away from yourself and firmly depress the canister to release 1 puff
- Check the dose indicator. If you are testing your inhaler for the first time, the indicator should read 200. The indicator counts down in intervals of 20.
Everyday use
Whenever possible, sit or stand in an upright position when using your inhaler.
To use your Clenil inhaler follow the 5 steps shown here. Do not rush yourself.
-
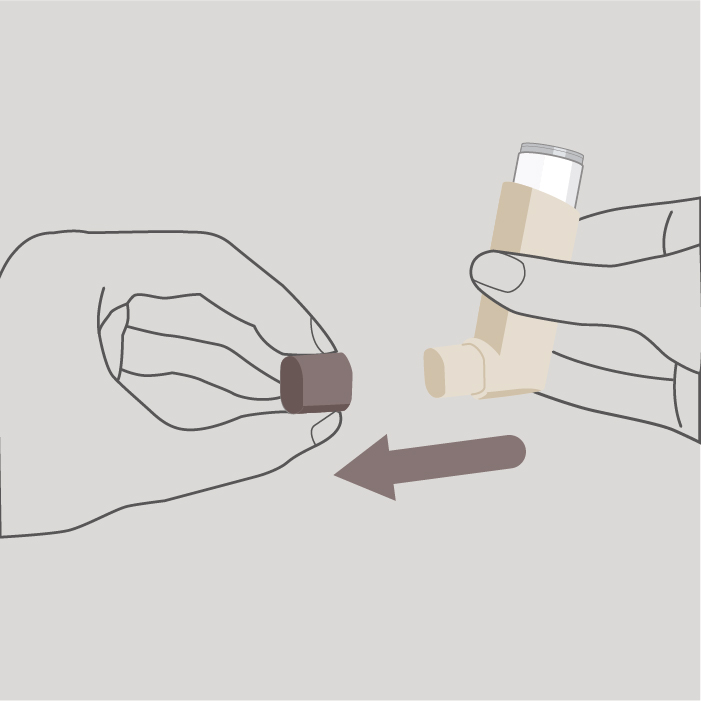
Step 1
Remove the protective cap from the mouthpiece and check that the mouthpiece is clean and free from dust, dirt or any foreign objects.
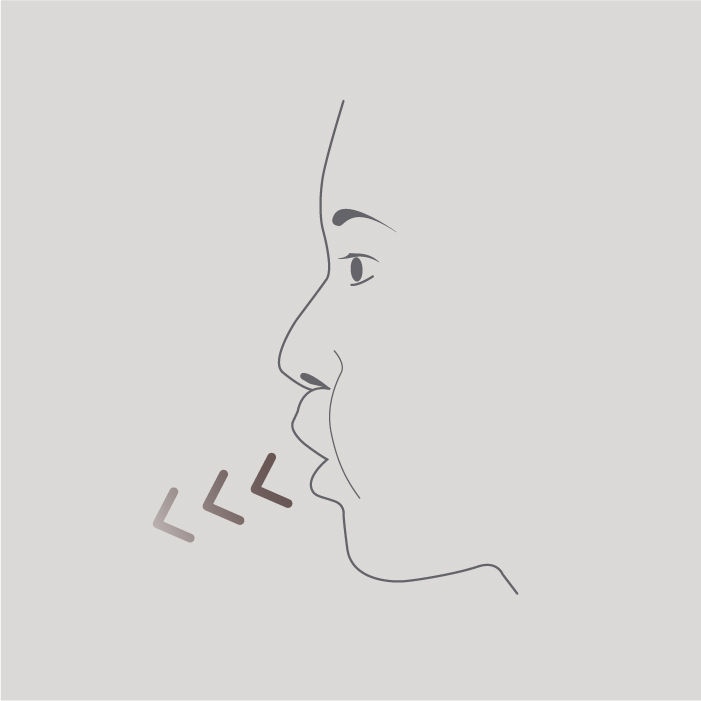
Step 2
Breathe out as far as is comfortable.
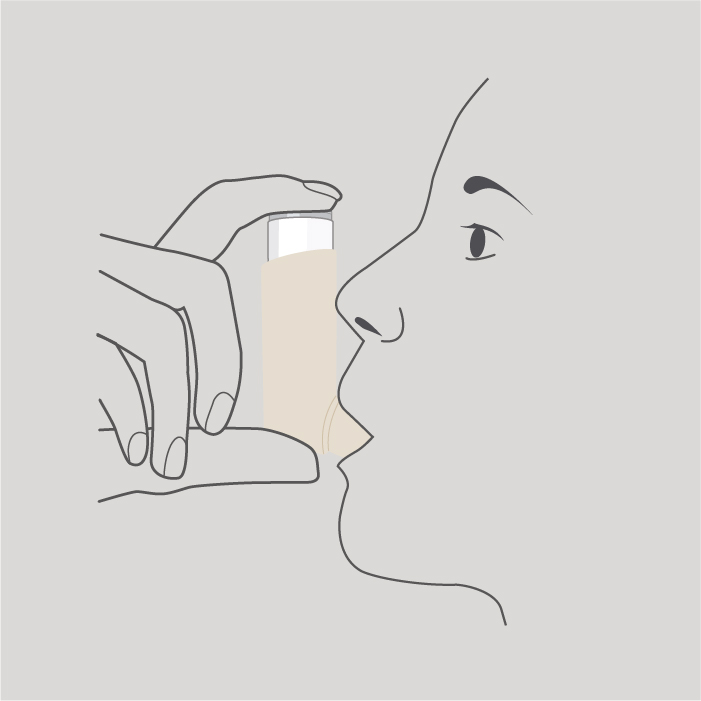
Step 3
Hold the inhaler upright as shown and put your lips around the mouthpiece.
Do not bite the mouthpiece.
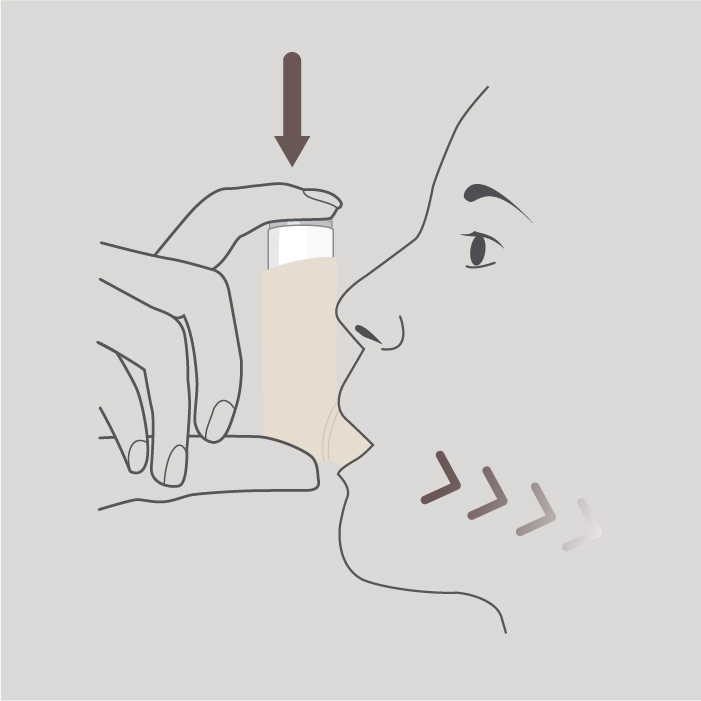
Step 4
Breathe in slowly and deeply through your mouth and, just after starting to breathe in, press down on the top of the inhaler to release 1 puff whilst continuing to breathe in slowly and deeply.
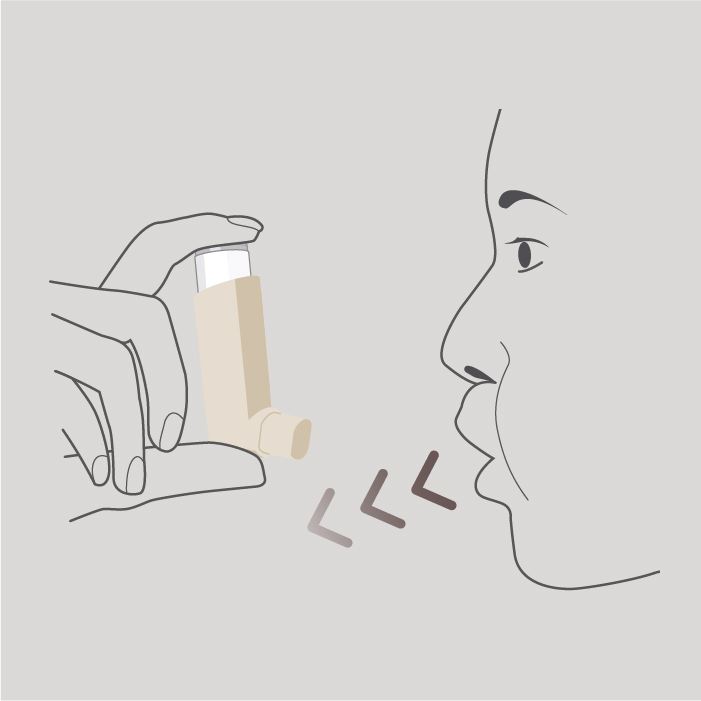
Step 5
Hold your breath for as long as is comfortable and, finally remove the inhaler from your mouth and breathe out slowly. Do not breathe into the inhaler.
If you take another puff, keep the inhaler upright and wait about half a minute before repeating steps 2 to 5.
Do not repeat steps 2 to 5 too quickly. After use, close your inhaler again with the mouthpiece cover.
See the information below about what to do after you have used your inhaler.
How to use Clenil with a spacer
Your doctor, nurse or pharmacist may have prescribed you a spacer, called a Volumatic™ Spacer to use with your inhaler.
Your spacer may look like a long chamber with a mouthpiece at one end and a hole at the other where it attaches to your inhaler.
Follow the next 9 steps to use your Clenil inhaler with a spacer. Don’t forget to refer to the information leaflet that comes with the Volumatic™ Spacer, or speak to your doctor, nurse or pharmacist for more information or help.
Before using your inhaler for the first time, or if it has not been used for 3 days or more, release 1 puff into the air to make sure it is working properly. Whenever possible, sit or stand in an upright position when using your inhaler.
-
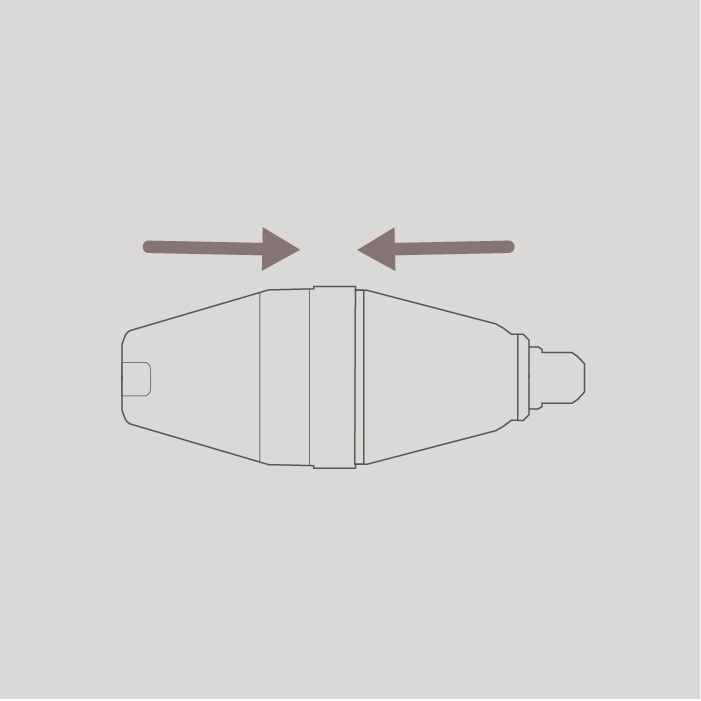
Step 1
Fit the 2 halves of the spacer together. Line up the notch on 1 half with the slot on the other, then press together.
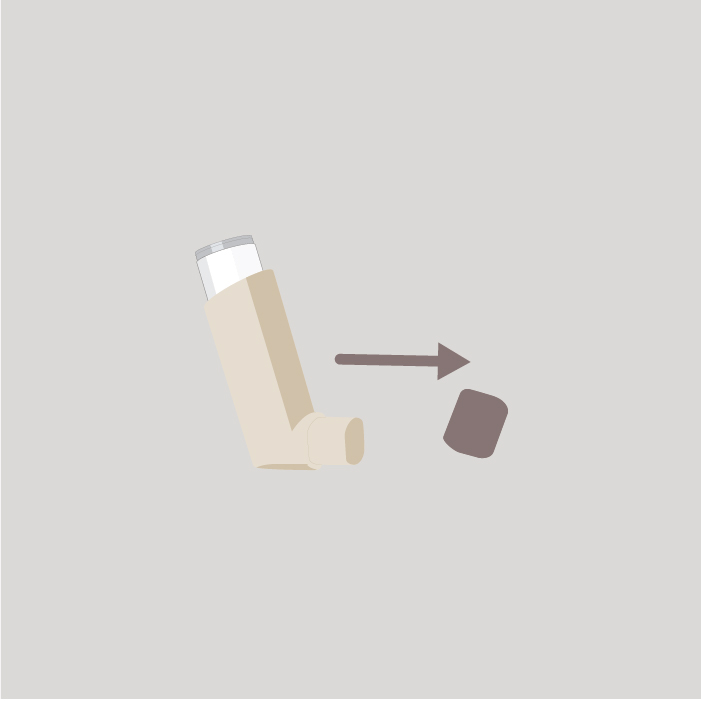
Step 2
Remove the protective cap from the mouthpiece and check that the mouthpiece is clean and free from dust, dirt or any foreign objects.
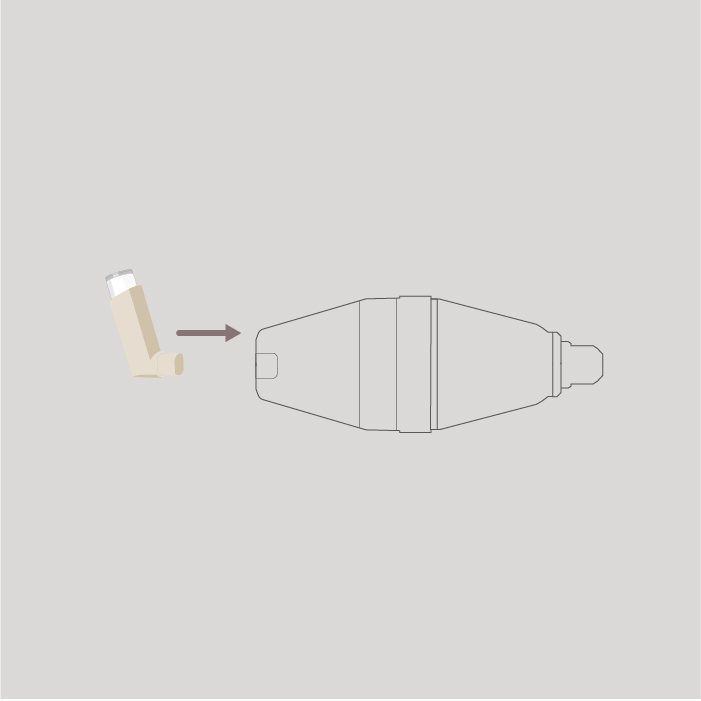
Step 3
Insert the mouthpiece of your inhaler into the flat end of the spacer.
Step 4
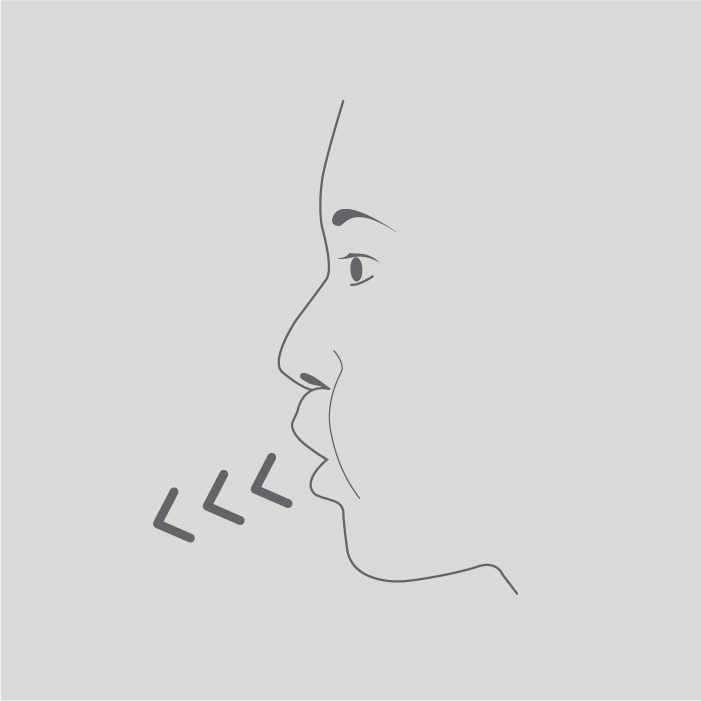
Breathe out as far as is comfortable.
Step 5
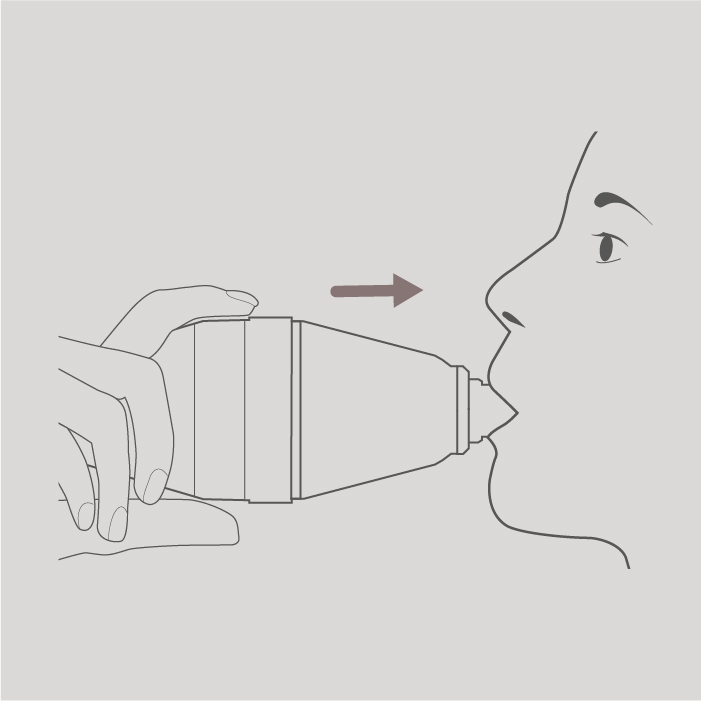
Put the mouthpiece of the spacer to your lips. Seal your lips around the mouthpiece. Do not bite the mouthpiece. Do not put your lips over the side holes of the mouthpiece.
Step 6
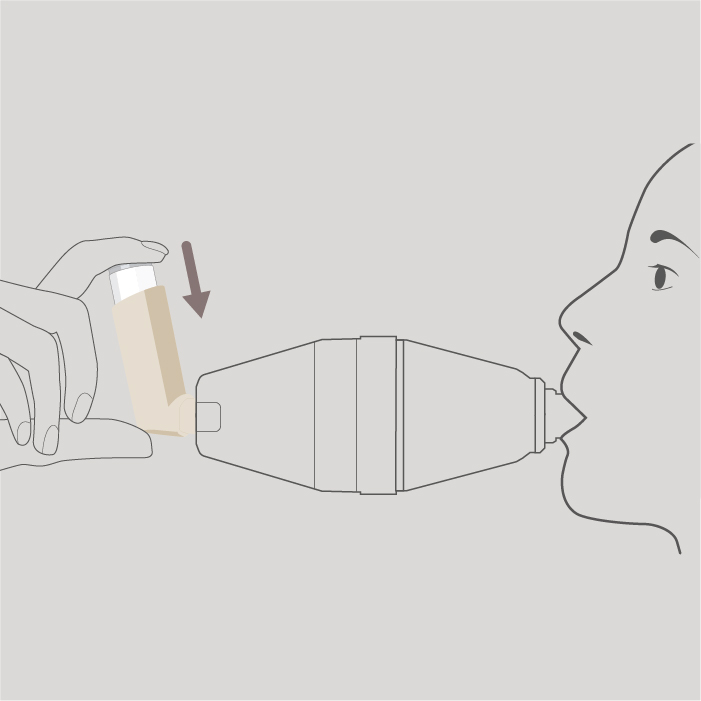
Press down on the top of your inhaler canister once to release 1 puff into the spacer.
Step 7
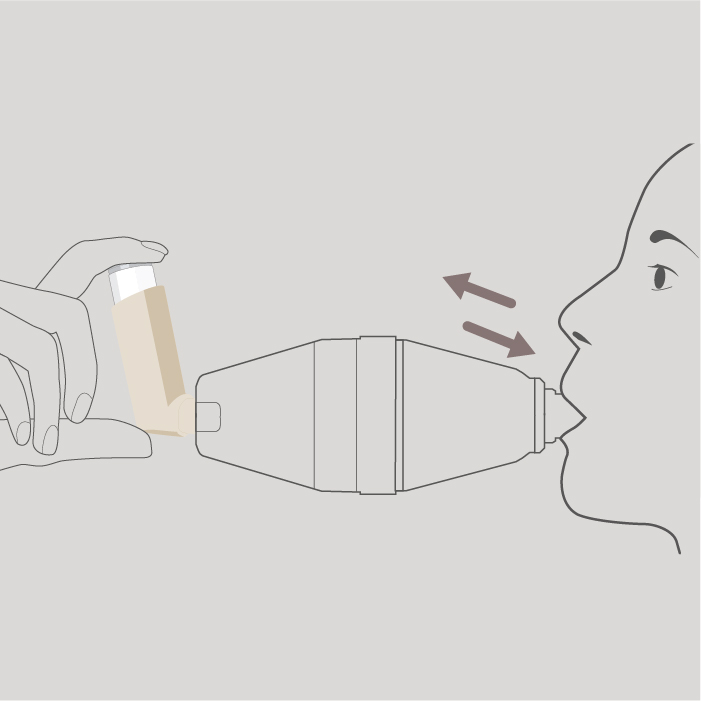
Take one deep steady breath or breathe normally in and out 5 times (tidal breathing). You should hear the mouthpiece valve ‘click’ or rattle as you breathe through it. If you don’t hear it, tilt the spacer up slightly and try again. Remove the spacer from your mouth.

Step 8
Wait 30 seconds and repeat steps 4-7 if a second puff is prescribed.

Step 9
Remove the inhaler from the spacer and replace the inhaler protective cap.
How to use Clenil
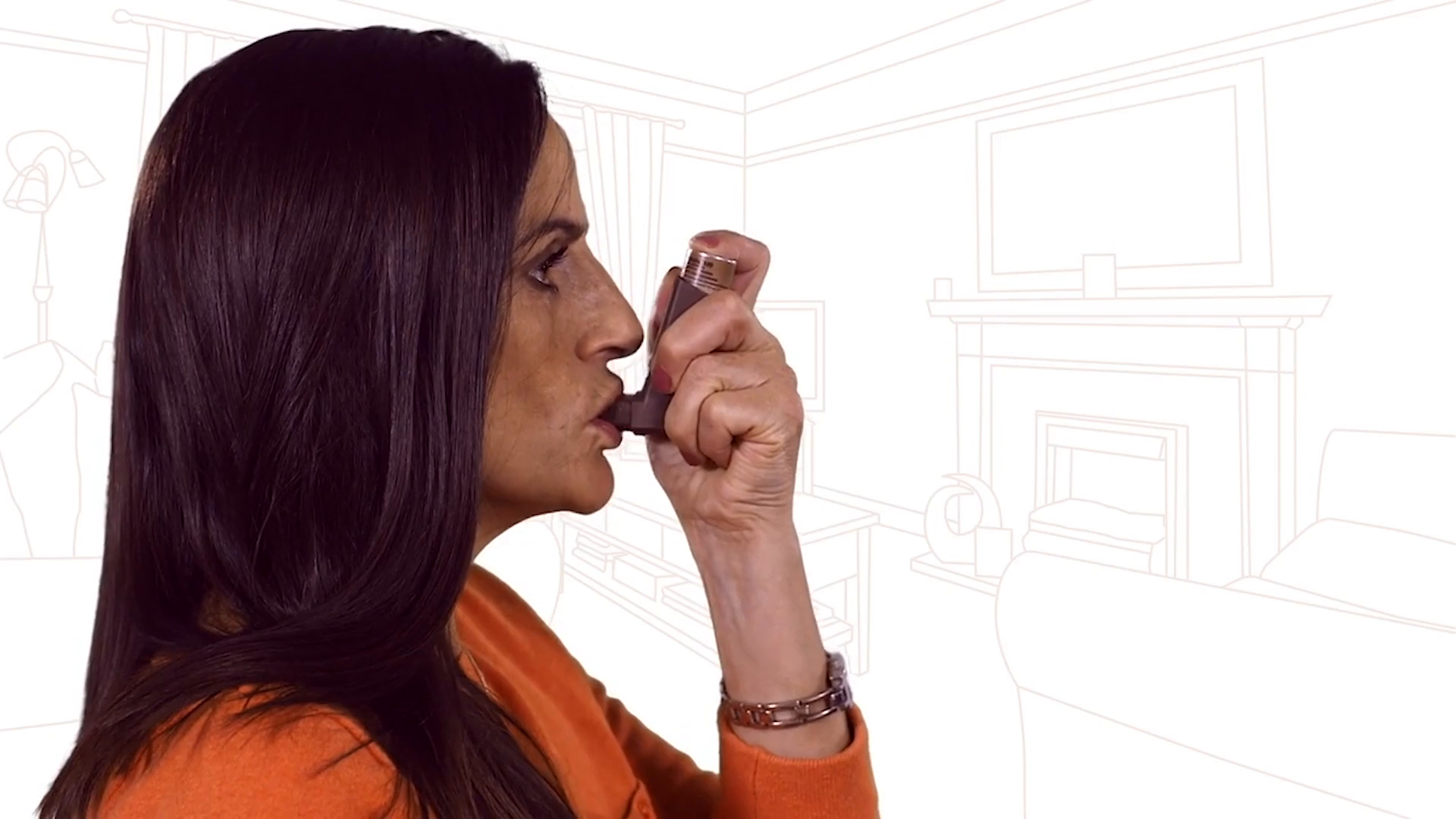
After use
To lower the risk of a fungal infection in your mouth and throat, rinse your mouth or gargle with water, without swallowing, or brush your teeth each time after you use your inhaler.
Replacements and disposal
You should get a replacement when the dose counter shows the number ‘20’. Stop using the inhaler when the dose counter shows ‘0’. This is because any medicine left in the inhaler may not be enough to give you a full dose.
Medicines should not be disposed of via waste water or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Frequently Asked Questions
Using your inhaler
-
Clenil is available in 4 different strengths (50 micrograms 100 micrograms 200 micrograms and 250 micrograms). Your doctor will have decided which strength you need. Always use your inhaler exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. It takes a few days for your inhaler to work. It is very important that you use it regularly.
-
Understandably, when you don’t have any symptoms, it can be easy to forget to take your treatment or to feel like you do not need to.
You must use your preventer inhaler every day, even when you are feeling well.
Regular use of your preventer inhaler is the most effective way to keep you feeling this way.
-
Step-by-step guides on how to use your inhaler are available in the Asthma User Guide.
-
Spacers are empty tubes that are usually made from plastic. They slot onto the mouthpiece of your inhaler on one end, and you use a mouthpiece or mask on the spacer at the other end. They help you get the best from your asthma medicine.1
Your doctor, nurse or pharmacist may have prescribed a spacer, called the Volumatic™ Spacer to be used with your inhaler. Your spacer may look like a long chamber with a mouthpiece at one end and a hole at the other where it attaches to your inhaler. See step-by-step instructions on how to use your inhaler with a spacer.
It is important that you read the package leaflet which is supplied with your Volumatic™ Spacer device, and that you follow the instructions on how to use and how to clean it, carefully.
Looking after your inhaler
-
Do not use Clenil after the expiry date which is stated on the carton and label. The expiry date refers to the last day of that month.
- Do not store the inhaler above 30°C
- Protect from frost and direct sunlight
- If the inhaler gets very cold, take the metal canister out of the plastic case and warm it in your hands for a few minutes before use. Never use anything else to warm it up
- Do not pierce the pressurised container.
-
When the indicator shows 20 doses left, you should request a new inhaler from your doctor. This will ensure you have a replacement in good time. When the ‘0’ appears centrally in the window the medicine has run out and you should discard the inhaler.
Your inhaler may feel like there are still some contents remaining but any remaining puffs may not contain enough medicine and should not be used.
-
It is important to clean your inhaler at least once a week, to stop it blocking up. For instructions on how to clean your inhaler, please refer to the Patient information leaflet included in the device packaging.
Side effects and other medication
-
Like all medicines, Clenil may cause side effects, although not everybody gets them. If you do get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the Patient Information Leaflet.
You can also report side effects directly via the Yellow Card Scheme. By reporting side effects you help provide more information on the safety of this medicine.
-
Your doctor, nurse or pharmacist can advise you about using other prescribed medications. You should also check with your pharmacist before taking medicines bought over the counter.
You must tell your doctor, nurse or pharmacist about any medication you may be taking, including herbal remedies.
Further resources and support
Find further information on living well with your asthma with our resources.
Asthma resources
Find further information and resources to help manage your asthma.
It can be isolating, suffering from a condition that may limit your ability to get out and about. There is a lot of information available online, but it’s important to go to a reliable source. The suggested resources listed below can offer you further support on the various aspects of living with asthma.
NHS
Helpline: 111 (non-emergency)
NHS asthma information
NHS Smokefree
Asthma + Lung UK
Helpline: 0300 222 5800 (non-emergency)
Explore support for asthma
Reference
-
- Spacers. Asthma + Lung UK. Available at: https://www.asthma.org.uk/advice/inhalers-medicines-treatments/inhalers-and-spacers/spacers/
The information provided here is not intended to replace the advice of your healthcare professional. If you have any questions about your medication, please speak to your doctor, nurse or pharmacist.
Volumatic™ is a registered trademark of the GlaxoSmithKline Group of Companies.
If you have not been prescribed Clenil and are looking for information on another Chiesi respiratory product please visit our homepage.
Reporting of side effects: If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.





